- +91-821-983-3540
- 01762-503026
- info@pharmaffairs.in
Green Field Projects
Home » Green Field Projects


Here at KP Pharmaffairs,we offer a comprehensive consultancy service for Greenfield Projects uniquely tailored for the pharmaceutical industry. Our professionals meticulously interact with clients to understand their goals, available resources, market trends, and formulate strategies for each and every component, from greenfield project planning to execution and commercialization.
Let KP Pharmaffairs be your partner as you boldly enter the energetic realm of pharmaceuticals with unrivalled confidence and clarity. Our consulting services in regard to greenfield projects assists new pharma manufacturers transform their visions into reality while ensuring sustainable success amid a volatile yet strategic marketplace.
Project Initiation & Conceptualization
- Feasibility Studies
Our extensive range of services here at KP Pharmaffairs includes the provision of detailed feasibility studies for projects considering aspects like market demand, legal requirements, and resource availability. As a baseline, we can provide our clients a detailed feasibility study based on:

Market Study
Product Selection, Assessment of the business environment, Supply and Demand forecasting, Market share analysis, Recommendations on product mix, Pricing & Distribution

Market Study
Product Selection, Business Environment Evaluation, Demand and Supply Projections, Market Share Evaluation, Recommendations on Pricing and Product Mix, Distribution Procedures, and Product Pricing

Technical Study
Preliminary Production & Equipment Capacities, Site Selection Studies, Project Cost Estimates, Technical Due Diligence, Project Timelines.

Technical Study
Assessments of the Preliminary Production and Equipment Capacities, Manufacturing facility Location Studies, Project Cost Estimates, Technical Due Diligence, Project Timelines.

Financial Study
CAPEX & OPEX, Financial Plan, Sales Cash Flow, Break Even Point, Profit & Loss Statement, Return on Investment.

Financial Study
Capital Expenditure and Operational Expenditure, Financial Structure, Revenue from Sales, Breakeven Analysis, Profit and Loss Statement, Return on Investment.
The feasibility study conducted under pharmaceutical projects is particularly useful for stakeholders as it allows them to identify the scope and limitations as well as the various risks associated with attempting to optimize a project.
Project Design and Planning
- Master Planning
A Master Plan concentrates on broad and long-term strategies for the development of Pharmaceutical Factories and Laboratories at a physical level. Helps in planning and anticipating potential higher growth with much ease, as it helps in estimating the scope of land utilization correctly. Hence, there is a direct relationship between land usage and organizational growth.
KP Pharmaffairs contracts the services of a top architectural and design firm to prepare a futuristic Master Plan with the aim of achieving:
- Best possible site for Utilities, Manufacturing, Administration, QC Laboratory, Research and Development Activities.
- Control of green and environmental concerns.
- Potential for enhancement of public and semi-public civic community facilities.
- Provisions for Plans and Policies for the future expansion and development.


- Concept Engineering
It represents a process aimed at determining contextual features and value metrics of a Pharmaceutical Manufacturing facility based on the client’s expressed and latent requirements. A particular scope of work and approach must be designed for each project with emphasis on project definition, data collection, verification, analysis, and decision making.
The first document in the project phases is called Concept Engineering and will act as the primary document of reference for Detailed Engineering. It begins with high level design of the project which is critical with respect to the rest of the deployment work that will be done later in the project.
The following items will be defined in the Concept Engineering documentation:
- Manufacturing facility Design Concept that incorporates varying international GMPs such as WHO, EU GMP, PIC/S, US FDA etc.
- Interaction Interface with International Process/Regulatory Authorities. People and Material Movements System.
- Schematic Representation of dynamic elements of System Materials, and Movement of Personnel.
- Balance of Utilities with Equipments.
- Estimates of costs and time required to accomplish the objectives.
- Concept Engineering include the following phases:
STAGE-1
This process is focused on gathering the functional requirements of the facility with regard to:
- Dosage Forms
- Batch Sizes
- Containment Needs
- Equipment and Machinery Type
- Inventory Norms
- Philosophy of Expansion
STAGE-2
This stage starts with concentrating on the issues of:
- Preliminary Layout concerning Space and Resources.
- Specific Cleanliness Environment Category Set.
- Optimum Water Quality and Water Treatment Facilities.
STAGE-3
From what has been collected so far, the information includes:
- Budget Allocated to Execute the Project.
- Facility Pre-Stage Layout. The preliminary layout should adhere to local and international accepted regulatory standards.
- Construction of Buildings in an Abstract Form.
- Form a User Requirement Specification (URS).
- Draws a schedule for the project using Gantt charts.
- Design the system for generation and distribution of water.
- Production environment designing like Heating, Ventilation, and Air Conditioning systems (HVAC)..
- Monitoring of the Pressure Differential.
Project Execution
- Basic Engineering
In the Basic Engineering phase, we at KP Pharmaffairs define the following aspects of the Project:
- Manufacturing facility layout options compliant to GMP.
- Comprehensive drawings depicting flow of men and material within the manufacturing facility in the approved layout
- Heating, Ventilating, and Air Conditioning (HVAC) System Design with Air Flow Diagram.
- Utility systems design: Water and Gases treatment, purification, filtration, distribution, steam and compressed air.
- Determine the electrical energy requirements of the manufacturing facility along with the designed high and low voltage power distribution schemes.

- Detailed Engineering
As the most advanced part of an engineering process, detailed engineering integrates construction with other factors, usually referred to as basic engineering. It is in this part that ‘dreams become a reality.’
In this part, we describe the steps taken in consideration to provide detailed engineering services for a project which are:
- Selection of the right equipment
- Electrical Engineering
- HVAC
- Planning of Instruments
- Control systems of Building Management and Automation
- Drafting of Detailed Specifications, Data Sheets and Bill of Quantities (BOQ) for various Parts, Equipment, Machinery and System bought.
- Buying of plant equipment, machinery, system etc.
- Plot Layout
- Area/Layout
- Men/Material
Movement, etc.
- Industrial Drainage
- Domestic Drainage, etc.
- Soil Drainage,etc.
- Illumination Design
- Lighting Layout, etc..
- Electrical Load Data
- Electrical Layouts
- Cabling Details
- Bill Of Quantities (BOQ), etc.
- HVAC Design
- Air Handling Unit (AHU)
Locations - Air Flow Diagrams
- Ducting Layouts
- BOQ, etc.
- Purified Water Generation System
- Water for Injection (WFI)
- Compressed Air
- Pure Steam
- Plant Steam

Civil Layouts
- Civil Map Planning includes Area/layout of the plot.
- Co-ordination of Man/Material Movement.

Drainage Layouts
- Domestic and industrial drainage layouts, Soil Drainage, etc.

Lighting
- Design of illumination systems.
- Layout of lighting for various areas.

Electrical
- Layout of data for Electrical Loads
- Power Distribution Diagrams
- Cabling Details
- Bill Of Quantities (BOQ).

HVAC (Heating Ventilation and Air Conditioning)
- Design of HVAC System; Locations of AHUs (Air Handling Units); Air Handling Unit or Ventilation Unit Flow Path Diagram; Ducts layout; Bill of Quantities- BOQ, etc.
Schematic Pipeline
- Pure Steam
- Manufacturing facility Steam (WFI)
- WFI (Water for Injection)
- Compressed Air
- Purified Water Generation System
Project Management
In our industry, project management is the discipline that focuses on the application of skills, knowledge, tools, and techniques to accomplish specific activities within a discipline to meet the customer’s needs and achieve the desired outcome in a timely and cost-effective manner. It is the most critical aspect in executing any project, because it keeps the project in line with its timelines.
- A complete and self-sufficient integrated project planning and control system is in place and functions at KP Pharmaffairs, which has a team of Project Management specialists.
- This Project Management staff will be able to note all the possible delays and slippages as well as the possible ways to achieve the project timelines. These delays may be due to Engineering drawings preparation by Design Consulting agency, Equipment delivery problems, Civil contractor work delays, or any other reasons.
- Project Management professional will interface with the vendor and other contractors to resolve the delays. KP Pharmaffairs has successfully executed 100+ projects in different regions within stringent time frames.
- With Project Management, KP Pharmaffairs has made life simple for the client by using Integrated approach to execute projects while controlling costs.
- In Project Management, KP Pharmaffairs will also advise design consulting agency to remedy any shortcomings in the designs that are in violation of cGMP International Drug Authorities norms such as US FDA, UK MHRA, TGA, EU GMP, PIC/s.

The corresponding Project Management Functions are the following:
- Construction of the Project Breakdown Structure.
- Project Time Schedule Preparation and Control.
- Cost estimate and development of Health and Safety provisions for the Detailed Engineering stage.
- Provide technical support to obtain the necessary administrative clearances.
- Availability check of Design Data.
- Project Documents Review
- Cost estimate and development of Health and Safety provisions for Detailed Engineering stage
- Attend regular client Progress review meetings, adjust client expectations vs. actual progress and remove constraints to progress.
- Mechanical Installation, Commissioning, and Validation.

Construction Management
KP Pharmaffairs has successfully implemented and executed more than 100 projects over the past years with the assistance of a highly skilled team. KP Pharmaffairs has always prided itself on its employee’s well-being and ergonomic safety. Remarkable employee security and satisfaction are prerequisites, which enables employees to always showcase their best passion and excellence. Health and safety conditions at the sites or offices are uncompromising. Owing to the strict adherence of work safety norms, KP Pharmaffairs has a record “ZERO” Hazards or deaths at any of the sites. Every employee lives up to this core value of health and safety.
During Construction Management, major emphasis is also given to EHS norms (Environment, Health & Safety).
The responsibilities of the Construction Management includes, but are not limited to:
- Scheduling and supervising the construction and installation activities, including the use of specialized tools and equipment
- Preparing weekly and monthly progress reports while providing feasible recommendations to expedite construction work.
- Logistics management of the project site.
- Monitoring changes and contractor’s activities for re-planning.
- Verification of invoices and other charges for work carried out.
- SHE (Safety, Hygiene and Environment) monitoring and controlling.
- Monitors the quality control of the material delivered on site and for each discipline against the scope of work.
- Monitors the provision of all design documents, and if they are not available, orders the necessary changes that satisfy the construction requirements.
- Conducts performance tests of the mechanical work on the site.
- Conducts and files the required documentation and submits results to the client. \n- As-Built documentation compilation.
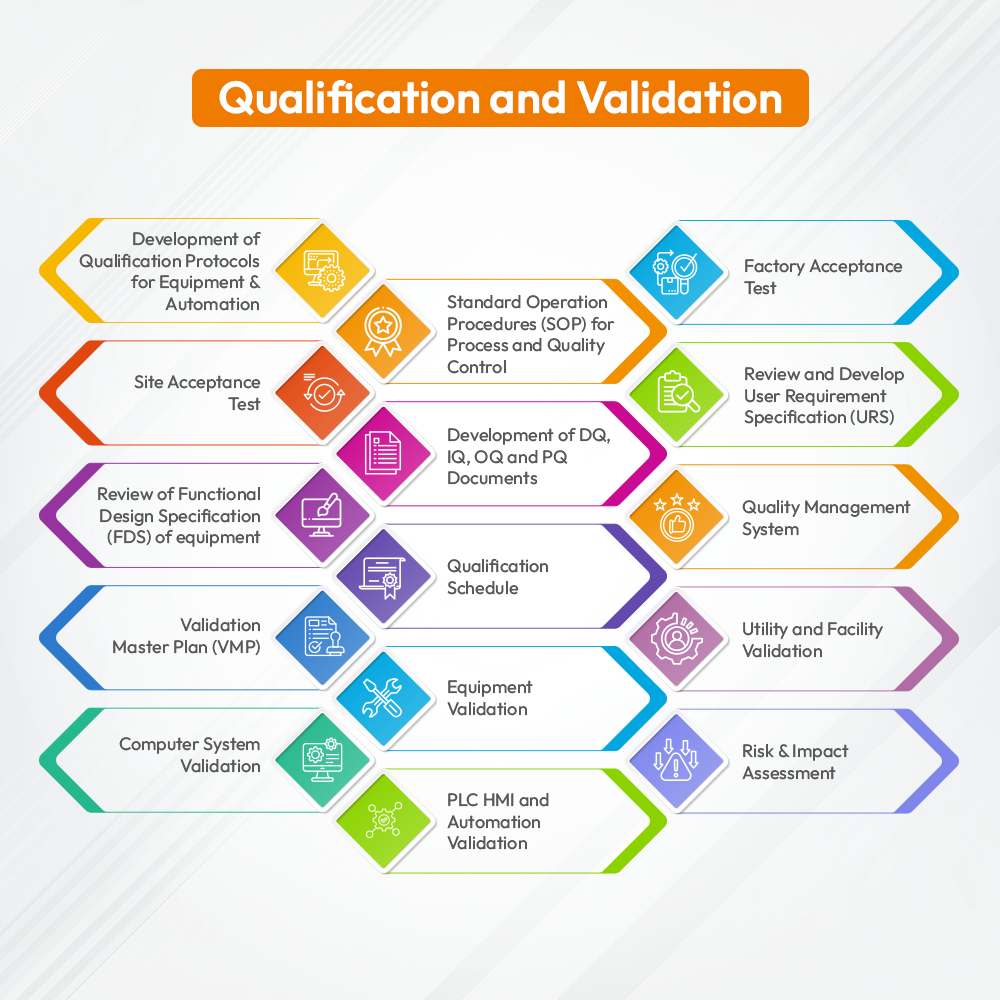
- Development of Qualification Protocols for Equipment & Automation
- Standard Operation Procedures (SOP) for Process and Quality Control
- Factory Acceptance Test
- Site Acceptance Test
- Development of DQ, IQ, OQ and PQ Documents
- Review and Develop User Requirement Specification (URS)
- Review of Functional Design Specification (FDS) of equipment
- Qualification Schedule
- Quality Management System
- Validation Master Plan (VMP)
- Equipment Validation
- Utility and Facility Validation
- Computer System Validation
- PLC HMI and Automation Validation
- Risk & Impact Assessment
- Development of Qualification Protocols for Equipment & Automation
- Standard Operation Procedures (SOP) for Process and Quality Control
- Factory Acceptance Test
- Site Acceptance Test
- Development of DQ, IQ, OQ and PQ Documents
- Review and Develop User Requirement Specification (URS)
- Review of Functional Design Specification (FDS) of equipment
- Qualification Schedule
- Quality Management System
- Validation Master Plan (VMP)
- Equipment Validation
- Utility and Facility Validation
- Computer System Validation
- PLC HMI and Automation Validation
- Risk & Impact Assessment
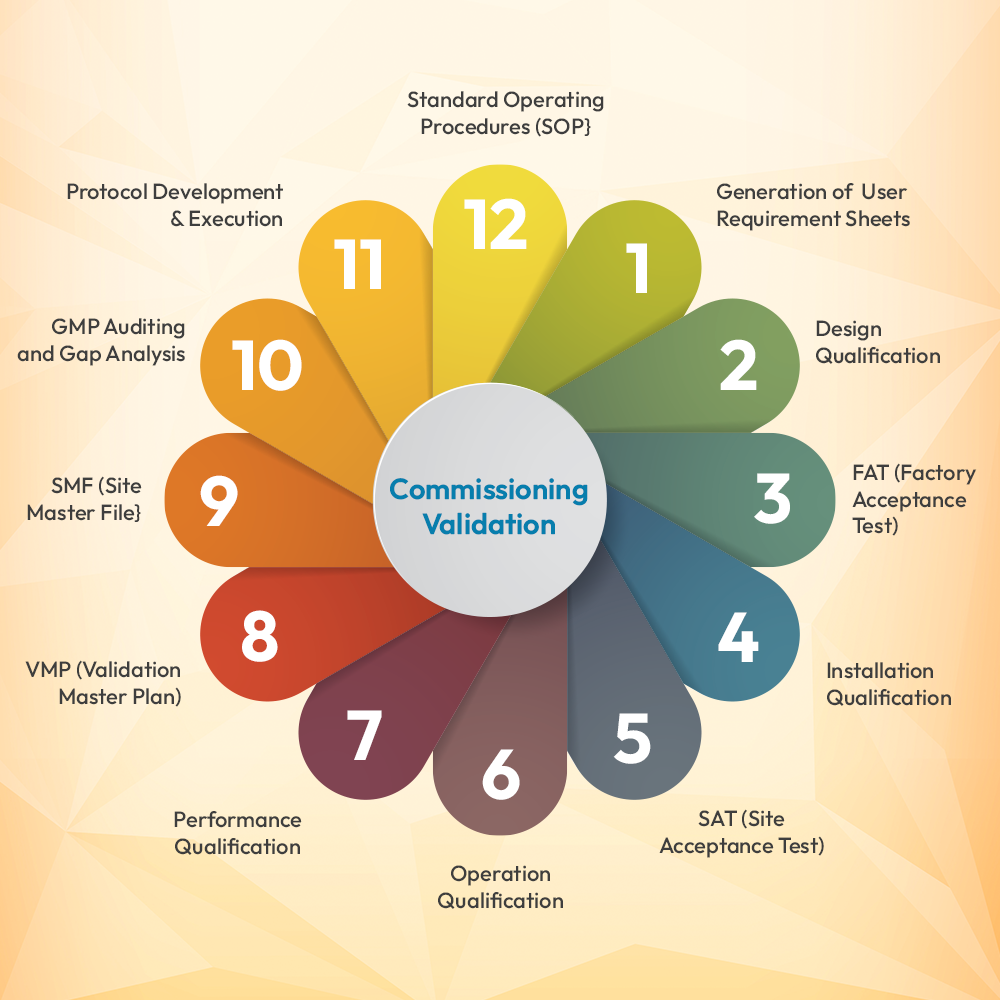
Commissioning Validation includes the following:
- Generation of User Requirement Sheets
- Design Qualification
- FAT (Factory Acceptance Test)
- Installation Qualification
- SAT (Site Acceptance Test)
- Operation Qualification
- Performance Qualification
- VMP (Validation Master Plan)
- SMF (Site Master File}
- GMP Auditing and Gap Analysis
- Protocol Development & Execution
- Standard Operating Procedures (SOP}
Project Commencement